The primary visual cortex has two main pathways into the parietal and temporal lobes. Select one of these pathways and, using empirical evidence, discuss its role in interpreting visual information.
The neurological basis for visual perception and recognition is one of our oldest scientific inquiries. Central to contemporary understandings are two distinct processing pathways that begin in the primary visual cortex: the dorsal pathway, which projects superiorly towards the parietal cortex, and the ventral pathway, projecting anteriorly towards the temporal cortex (see Figure 1). These pathways serve separate functions, the ventral pathway for object recognition, and the dorsal pathway for spatial perception and action coordination. In this essay, the focus is exclusively on the former. It will first evaluate the empirical evidence highlighting the specific role of the ventral pathway in the coding of visual inputs into semantic representations. It will then outline the current hierarchical model that is used to conceptualise how the ventral pathway processes visual information, as well as the emerging criticisms of this idea. Finally, this essay seeks to challenge the dorsal-ventral dichotomy presupposed by this question, arguing that any attempt to ‘select one pathway’ undermines their collaborative contributions to visual information processing.
Figure 1
Dorsal and ventral pathways extending from V1 into parietal and temporal lobes.
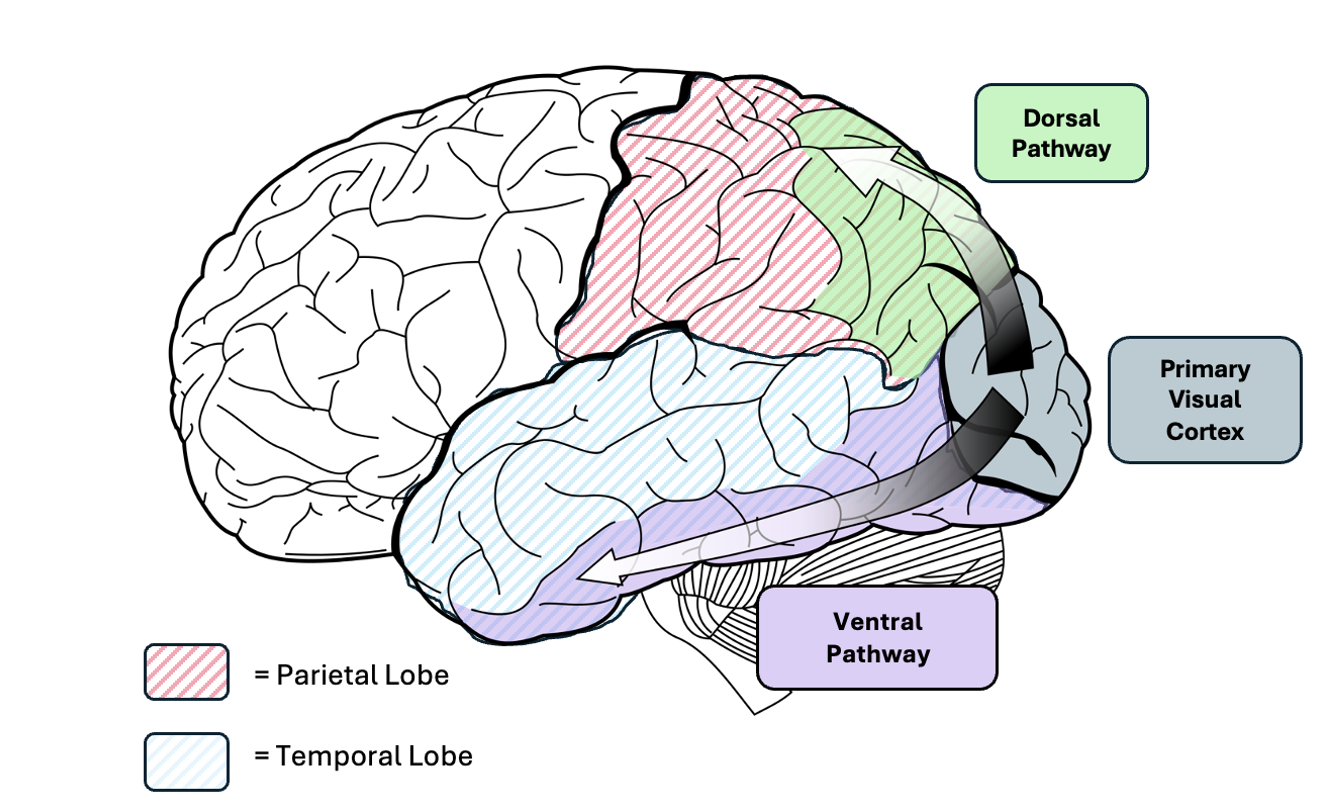
The ventral pathway is now recognised as crucial for interpreting visual information through the translation and encoding of visual inputs into meaningful semantic interpretations. Initially, lesion studies on macaques highlighted the role of ventral-pathway associated areas in object recognition (Ungerleider & Mishkin, 1982). Clinical studies have shed further light on the pathway’s specific function. After patient D.F. suffered bilateral lesions in the lateral occipital cortex (LOC) following carbon monoxide poisoning, behavioural studies demonstrated that, while the impairment of this area of the ventral pathway left her unable to recognise and identify objects, she maintained sensitive visuomotor coordination, the ability to draw familiar objects from memory, as well as discern between an image and its scrambled version (Goodale et al., 1991, 1994; James et al., 2003). These observations demonstrated the specific role of the ventral pathway in transforming visual inputs into semantic recognition. The generalisability of this conclusion is reinforced by other agnosia patients showing similar object recognition impairments following ventral pathway lesions, such as H.J.A. (Riddoch & Humphreys, 1987), M.S. (Zeki, 1990) and S.B. (Lê et al., 2002), as well as in meta-analyses of this relationship (Grill-Spector et al., 2001; Bridge et al., 2013).
Having established the importance of the ventral pathway in object recognition, the mechanisms by which it does so remain to be understood. Two ideas govern current understandings: first, that various regions within the pathway perform specialised functions, and second, although facing increasing challenges, that the pathway follows a hierarchical progression in processing visual inputs from the primary visual cortex (V1) to the inferior temporal cortex (IT).
Regarding the former, robust empirical evidence supports the functional specialisation of regions in the ventral pathway. For instance, the fusiform face area (FFA) consistently exhibits higher neural activity in response to faces compared to non-face objects, and when damaged results in prosopagnosia, an impairment in the ability to recognise faces (Kanwisher et al., 1997; Witthoft et al., 2016). Similarly, distinct patterns of stimuli-specific increased neuronal activity characterise other regions of the pathway, such as the parahippocampal place area in response to scenes and buildings (Epstein & Kanwisher, 1998) the extrastriate body area (EBA) in response to body parts (Downing et al., 2001), and the anterior and posterior collateral sulci in response to colours and textures (Cavina-Pratesi et al., 2010). Disruptions to these areas through transcranial magnetic stimulation show specific impairments in the processing of associated stimuli (e.g., Parvizi et al., 2012; Pitcher et al., 2014). Developmental studies have noted parallels between the developments of specific infant capacities for visual recognition and the maturation of associated ventral pathway structures (Grill-Spector et al., 2008; Golarai et al., 2007).
Regarding the latter, the traditional hierarchical model of ventral pathway processing suggests that visual inputs received in V1 undergo a sequential series of processing stages that build increasingly complex object representations by integrating outputs from earlier stages (DiCarlo et al., 2012). This idea was initially proposed based on electrophysiological findings indicating that neurons in more rostral regions of the pathway respond to more complex visual stimuli than neurons in caudal regions (Hubel & Wiese, 1962). Newer electrophysiological evidence has substantiated these claims. While the lateral occipital cortex (LOC) responds invariantly to image visual stimuli, V4 exhibits much more selective responses to various object stimuli transformations (e.g., Tsunoda et al., 2001; Serre et al., 2007; Bussey & Saksida, 2002). Similarly, lesions in posterior areas of the ventral pathway produce complete blindness in part of the visual field (Stoerig & Cowey, 1997), while lesions in anterior regions produce more selective deficits in the ability to recognise and classify complex objects (Holmes & Gross, 1984; DiCarlo et al., 2012). As such, the notion of a linear, hierarchical model has become dominant in current understandings of the ventral pathway.
However, new evidence challenges the hierarchical model by suggesting a more recurrent processing model. Four ideas have emerged at the forefront of these criticisms. First, direct communication between supposed ‘early’ and ‘late’ stages of the hierarchy undermines the unidirectionality of this model. Neuroanatomical studies have revealed that V1 projects directly not just to V2, but also to V3, V4, and the medial temporal lobe, thus violating the linearity of the hierarchical model (Van Essen et al., 1986, Ungerleider et al., 2008). Second, researchers have now observed the direct, and nonreciprocal projections from so-called ‘late’ stages back to ‘early’ stages, indicating a feedback rich connectivity pattern (Kravitz et al., 2013). Third, the hierarchical model implies stronger serial connections between regions of the ventral pathway (e.g., V1 to V2, V2 to V3) compared to long-range connections (e.g., V1 to V3, V1 to V6). However, at present there is little direct evidence for this assumption, and human and animal studies have revealed neuronal connections inconsistent with this linear pathway organisation (deHaan & Cowey, 2011; Sing & Modha, 2010). Finally, the hierarchical model suggests systematic and linear increases in processing complexity along the ventral pathway. However, newer research has demonstrated that the processing of so-called ‘simple’ percepts, such as brightness and colour, shares many characteristics with the processing of more ‘complex’ percepts, such as words and faces (deHaan & Cowey, 2011). Given these challenges to the hierarchical model, recognising the ventral pathway as a dynamic, interconnected network may offer a better understanding of how it processes visual information.
While the conceptual division of ventral and dorsal pathways has provided a useful framework for understanding visual processing, the notion of cleanly separating these pathways into distinct entities is undergoing increasing reconsideration. Emerging evidence suggests a more nuanced interaction between the two pathways, as highlighted by neuroimaging studies demonstrating extensive crosstalk (Schenk & McIntosh, 2010). Furthermore, fMRI studies have revealed activations in the opposing pathway during tasks traditionally attributed solely to the other pathway (Zacahariou et al., 2014; Konen & Kastner, 2008). Notably, these studies represent a departure from previous methodologies by examining ventral-dorsal activation without the confounds of working memory activation or reach/grasp responses. Coupled with findings that ventral-dorsal activation is highly affected by stimuli and task choice, it appears that previous observations of a strict ventral-dorsal dichotomy may have been influenced by specific experimental conditions (Erlikhman, 2018).
As such, an emerging perspective suggests the composition of the ventral pathway, not merely as a singular ‘what’ pathway, but rather, as the collective outcome of processing from ‘what’-associated regions (Freud et al., 2016, 2020). These nuances may be understood in the case of patient D.F., whose case has been pivotal in arguments supporting a ventral-dorsal dichotomy. Initial studies contrasted her symptoms with those of optic ataxia, which involves lesions to the dorsal pathway, using these comparisons to establish the specific and functionally dissociated roles of the pathways. However, recent fMRI studies have shown that the responses of D.F. to dorsal pathway-associated cues are impaired compared to healthy controls, indicating that the latter are less susceptible to such manipulations due to additional visual cues processed by the ventral pathway (Goodale et al., 2008). This critique may be extended to other agnosia case studies, particularly when considering that the majority, including D.F., involve brain damage due to carbon monoxide poisoning (CO; Karnath et al., 2009). CO intoxication leads to a diffuse and widespread pattern of neuronal damage throughout the brain, complicating interpretations of the specific role of the ventral pathway (Betterman & Patel, 2014). Severe perceptual impairments may have arisen, not solely from damage to the ventral (or dorsal) pathway, but from broader damage to a number of ventral pathway-associated regions, some potentially in the supposedly distinct visual cortical pathway.
To conclude, it is clear that the ventral pathway is crucial in interpreting visual information by recognising the varying attributes of stimuli and synthesising inputs into a coherent perception, essentially telling us ‘what’ we are looking at. However, the precise manner in which it does so is yet to be definitively confirmed. Traditional views propose a hierarchical, linear model of increasingly complex stimulus recognition along the pathway. However, this model appears reductive in light of newer evidence, and future research would benefit from considering the interconnectedness of pathway stages, and the prevalence of feedback loops. Furthermore, while the conventional ‘dorsal-ventral’ dichotomy, presupposed in the question at hand, provides a useful framework through which to understand visual perception, the notion that they can be neatly separated into mutually exclusive domains is doubtful. In reality, interpreting visual stimuli involves a fluid, task-specific combination of dorsal and ventral pathways, in which the dominant role of either is determined contextually. Studying the visual perception of the ventral pathway requires considering it not as function of a singular ‘what’ pathway, but as the joint outcome of the processing and coordination of various ‘what’-associated regions that may or may not involve both cortical visual pathways.
References
Betterman, K., & Patel, S. (2014). Neurologic complications of carbon monoxide intoxication. Handbook of clinical neurology, 120, 971-979.
Bridge, H., Thomas, O. M., Minini, L., Cavina-Pratesi, C., Milner, A. D., & Parker, A. J. (2013). Structural and functional changes across the visual cortex of a patient with visual form agnosia. Journal of Neuroscience, 33(31), 12779-12791.
Bussey, T. J., & Saksida, L. M. (2002). The organization of visual object representations: a connectionist model of effects of lesions in perirhinal cortex. European Journal of Neuroscience, 15(2), 355-364.
Cant, J. S., Arnott, S. R., & Goodale, M. A. (2009). fMR-adaptation reveals separate processing regions for the perception of form and texture in the human ventral stream. Experimental Brain Research, 192, 391-405.
Cavina-Pratesi, C., Kentridge, R. W., Heywood, C. A., & Milner, A. D. (2010). Separate channels for processing form, texture, and color: evidence from fMRI adaptation and visual object agnosia. Cerebral cortex, 20(10), 2319-2332.
de Haan, E. H., & Cowey, A. (2011). On the usefulness of ‘what’and ‘where’pathways in vision. Trends in cognitive sciences, 15(10), 460-466.
DiCarlo, James J., Zoccolan, D., & Rust, Nicole C. (2012). How Does the Brain Solve Visual Object Recognition? Neuron, 73(3), 415–434. https://doi.org/10.1016/j.neuron.2012.01.010
DiCarlo, J. J., Zoccolan, D., & Rust, N. C. (2012). How does the brain solve visual object recognition?. Neuron, 73(3), 415-434.
Downing, P. E., Jiang, Y., Shuman, M., & Kanwisher, N. (2001). A cortical area selective for visual processing of the human body. Science, 293(5539), 2470-2473.
Epstein, R., & Kanwisher, N. (1998). A cortical representation of the local visual environment. Nature, 392(6676), 598-601.
Erlikhman, G., Caplovitz, G. P., Gurariy, G., Medina, J., & Snow, J. C. (2018). Towards a unified perspective of object shape and motion processing in human dorsal cortex. Consciousness and cognition, 64, 106-120.
Freud, E., Behrmann, M., & Snow, J. C. (2020). What does dorsal cortex contribute to perception?. Open Mind, 4, 40-56.
Freud, E., Plaut, D. C., & Behrmann, M. (2016). ‘What’is happening in the dorsal visual pathway. Trends in Cognitive Sciences, 20(10), 773-784.
Golarai, G., Ghahremani, D. G., Whitfield-Gabrieli, S., Reiss, A., Eberhardt, J. L., Gabrieli, J. D., & Grill-Spector, K. (2007). Differential development of high-level visual cortex correlates with category-specific recognition memory. Nature neuroscience, 10(4), 512-522.
Goodale, M. A., Jakobson, L. S., Milner, A. D., Perrett, D. I., Benson, P. J., & Hietanen, J. K. (1994). The nature and limits of orientation and pattern processing supporting visuomotor control in a visual form agnosic. Journal of Cognitive Neuroscience, 6(1), 46-56.
Goodale, M. A., Milner, A. D., Jakobson, L. S., & Carey, D. P. (1991). A neurological dissociation between perceiving objects and grasping them. Nature, 349(6305), 154-156.
Grill-Spector, K., Golarai, G., & Gabrieli, J. (2008). Developmental neuroimaging of the human ventral visual cortex. Trends in cognitive sciences, 12(4), 152-162.Chicago
Grill-Spector, K., Kourtzi, Z., & Kanwisher, N. (2001). The lateral occipital complex and its role in object recognition. Vision research, 41(10-11), 1409-1422.
Holmes, E. J., & Gross, C. G. (1984). Effects of inferior temporal lesions on discrimination of stimuli differing in orientation. Journal of Neuroscience, 4(12), 3063-3068.
Hubel, D. H., & Wiesel, T. N. (1962). Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. The Journal of physiology, 160(1), 106.
Jacques, C., Witthoft, N., Weiner, K. S., Foster, B. L., Rangarajan, V., Hermes, D., … & Grill-Spector, K. (2016). Corresponding ECoG and fMRI category-selective signals in human ventral temporal cortex. Neuropsychologia, 83, 14-28.
James, T. W., Culham, J., Humphrey, G. K., Milner, A. D., & Goodale, M. A. (2003). Ventral occipital lesions impair object recognition but not object‐directed grasping: an fMRI study. Brain, 126(11), 2463-2475.Chicago
Kanwisher, N., Woods, R. P., Iacoboni, M., & Mazziotta, J. C. (1997). A locus in human extrastriate cortex for visual shape analysis. Journal of Cognitive Neuroscience, 9(1), 133-142.
Karnath, H. O., Rüter, J., Mandler, A., & Himmelbach, M. (2009). The anatomy of object recognition—visual form agnosia caused by medial occipitotemporal stroke. Journal of Neuroscience, 29(18), 5854-5862.
Konen, C. S., & Kastner, S. (2008). Two hierarchically organized neural systems for object information in human visual cortex. Nature neuroscience, 11(2), 224-231.
Kravitz, D. J., Saleem, K. S., Baker, C. I., Ungerleider, L. G., & Mishkin, M. (2013). The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends in cognitive sciences, 17(1), 26-49.Chicago
Lê, S., Cardebat, D., Boulanouar, K., Hénaff, M. A., Michel, F., Milner, D., … & Démonet, J. F. (2002). Seeing, since childhood, without ventral stream: a behavioural study. Brain, 125(1), 58-74.
Mishkin, M., & Ungerleider, L. G. (1982). Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behavioural brain research, 6(1), 57-77.
Modha, D. S., & Singh, R. (2010). Network architecture of the long-distance pathways in the macaque brain. Proceedings of the National Academy of Sciences, 107(30), 13485-13490.Chicago
Parvizi, J., Jacques, C., Foster, B. L., Withoft, N., Rangarajan, V., Weiner, K. S., & Grill-Spector, K. (2012). Electrical stimulation of human fusiform face-selective regions distorts face perception. Journal of Neuroscience, 32(43), 14915-14920.
Pitcher, D., Duchaine, B., & Walsh, V. (2014). Combined TMS and fMRI reveal dissociable cortical pathways for dynamic and static face perception. Current Biology, 24(17), 2066-2070.Chicago
Riddoch, M. J., & Humphreys, G. W. (1987). A case of integrative visual agnosia. Brain, 110(6), 1431-1462.
Schenk, T., & McIntosh, R. D. (2010). Do we have independent visual streams for perception and action?. Cognitive Neuroscience, 1(1), 52-62.
Serre, T., Oliva, A., & Poggio, T. (2007). A feedforward architecture accounts for rapid categorization. Proceedings of the national academy of sciences, 104(15), 6424-6429.
Stoerig, P., & Cowey, A. (1997). Blindsight in man and monkey. Brain: a journal of neurology, 120(3), 535-559.
Tsunoda, K., Yamane, Y., Nishizaki, M., & Tanifuji, M. (2001). Complex objects are represented in macaque inferotemporal cortex by the combination of feature columns. Nature neuroscience, 4(8), 832-838.
Ungerleider, L. G., Galkin, T. W., Desimone, R., & Gattass, R. (2008). Cortical connections of area V4 in the macaque. Cerebral Cortex, 18(3), 477-499.
Van Essen, D. C., Newsome, W. T., Maunsell, J. H. R., & Bixby, J. L. (1986). The projections from striate cortex (V1) to areas V2 and V3 in the macaque monkey: asymmetries, areal boundaries, and patchy connections. Journal of Comparative Neurology, 244(4), 451-480.
Zachariou, V., Klatzky, R., & Behrmann, M. (2014). Ventral and dorsal visual stream contributions to the perception of object shape and object location. Journal of Cognitive Neuroscience, 26(1), 189-209.
Zeki, S. (1990). A century of cerebral achromatopsia. Brain, 113(6), 1721-1777.
